Menu
Protocols
In-Gel Sample Preparation Guidelines
The quality of sample extraction and preparation significantly impact MS results. For gel bands, please carefully follow the following instructions:
- CRITICAL: Special care must be taken to avoid contamination in every step, especially with keratins from skin or hair (always wears clean nitrile gloves and work in a dust- free environment).
- CRITICAL: DO NOT use silverstaining! Only Coomassie-stained gels!
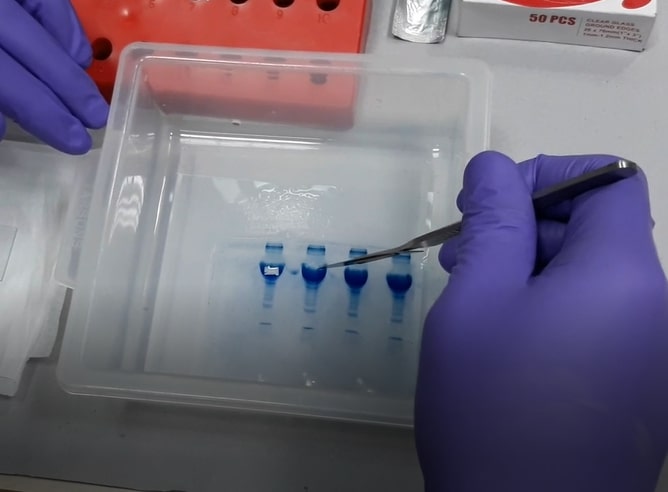
- CRITICAL: PRECISELY cut out ONLY the band of interest (only the stained area) - any excess gel will lead to background noise. If the band is extremely faint, there is a likely chance that we won't get any protein identification. So, we suggest you load the maximum possible amount of protein sample into the gel so that we get a fairly visible band with Coomassie stain.
- Email us a gel picture before sending the samples. This will help us to understand whether the band is properly stained or not. This could also be used as a reference to understand the sample concentration and its complexity. Since the nano-LC is a highly sensitive instrument, it is very important not to overload the nano-LC column with excess sample for the optimal results.
- ONLY cut gels on a clean glass plate (never use overhead projector foils or aluminium foils). The glass plate can be cleaned by using organic solvents like 70% ethanol or methanol.
- Use a NEW clean scalpel blade for precise cutting of gel spots.
- Always use filtered deionized water.
- DO NOT wash any flasks, tubes, or glass plates for electrophoresis with soap (or any polymeric detergent). Always, rinse your glassware with hot water and then an organic solvent like 70% ethanol or methanol.
- Freshly prepare all the buffers and stains needed to run the gel for MS analysis (DO NOT re-use).
- Place the gel cubes in a clean microcentrifuge tube (1.5 ml) and add enough methanol (50% methanol) in it to cover the gel cubes. The addition of large volumes of the buffer is not required, having them moist is enough.
- Make sure that the lid of the microcentrifuge tube is CLOSED PROPERLY before placing it in an envelope to send.
- DO NOT use parafilm around the lids.
- You can send the samples by courier or ordinary post. There is no need to ship the samples on ice. The samples are stable in 50% methanol.
In-Gel Sample Digestion Protocol
Reagents required
| 1 | Ammonium bicarbonate (Sigma A6141) | working concentration 50 mM |
| 2 | Dithiothreitol (Sigma D5545) | working concentration 100 mM |
| 3 | Iodoacetamide (Sigma I1149) | working concentration 200 mM |
| 4 | MS grade Trypsin (Sigma T6567) | working concentration 13 ng /μl |
| 5 | Formic acid (Merck 5.33002.0050) | |
| 6 | Acetonitrile (Merck 1.00029.2500) |
Reagent Setup (prepare freshly before use)
| 1 | Destaining solution: 100 mM ammonium bicarbonate/ acetonitrile (1:1, v/v) |
| 2 | Formic acid 5% in milliQ water (v/v). |
| 3 | Extraction buffer: 5% formic acid/acetonitrile (1:2, v/v) |
| 4 | Trypsin buffer: 13 ng /μl trypsin in 50 mM ammonium bicarbonate. |
Procedure
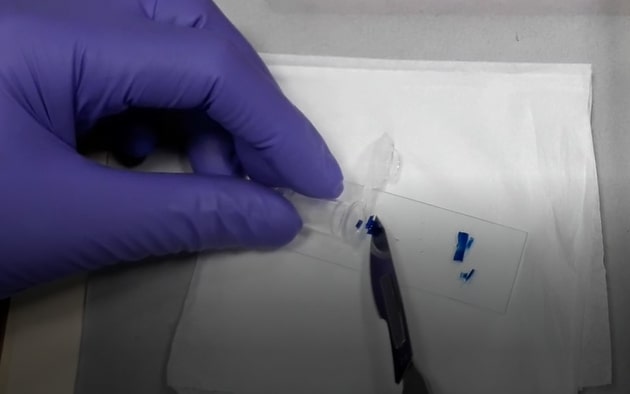
- Wipe the glass slides, scalpel with methanol. Excise the protein band from acrylamide gel with the scalpel on a glass slide avoiding the unstained gel region.
- Chop excised bands into small cubes of 1 x 1 mm size and transfer the pieces into duly labelled 1.5 ml centrifuge tubes.
- Rinse the gel bands with milliQ water for 1 hour by vortexing @1500rpm
- Discard the water and repeat the rinsing step for another 1 hour with 1 ml of fresh milliQ water.
- Spin gel cubes at 3000 rpm for 20 seconds and remove the water completely.
- Add 300 μl of destaining solution and vortex for 30 minutes @1500 rpm, depending on the staining intensity.
- Add 500 μl of 100% acetonitrile and incubate at room temperature with vortexing for 10-15 minutes or until the gel pieces become white and shrink.
- Remove acetonitrile completely and keep the vials open for 20 minutes for the solvent to evaporate completely.
- Add 30-50 μl of 13 ng/μl trypsin buffer in such a way that it covers the dry gel pieces and leave it in an ice bucket or a fridge for saturating the gel.
- After 30 minutes, check the level of trypsin buffer and if necessary, add more trypsin buffer for the gel pieces to be completely covered with trypsin buffer during the digestion step.
- Leave the gel pieces for another 90 minutes to saturate them with trypsin buffer. Check the levels of trypsin buffer in the vials again to see if the gels are remaining submerged. If needed, add 10-20μl of ammonium bicarbonate buffer so that the gel pieces remain wet during enzymatic cleavage..
- Incubate the vials at 37°C at 300 rpm overnight with gentle vortexing.
- Remove the trypsin buffer from the sample vials to respective freshly labelled 0.6 ml of microcentrifuge tubes.
- Add the extraction buffer (twice the volume of trypsin buffer used initially, usually 100μl) to the sample tubes containing gel pieces and vortex vigorously for 20 minutes.
- Aspirate the extraction buffer and combine it with the 0.6 ml microcentrifuge tubes.
- Dry the entire samples using Speed Vacuum concentrator and store at -20 until use.
- Finally reconstitute the samples in 30 μl of 3% acetonitrile before the LC/MS analysis.
In-Solution Sample Preparation Guidelines
The quality of sample extraction and preparation significantly impact MS results. For in-solution samples, strictly follow the following guidelines:
- DO NOT wash any flasks, tubes, or glass plates (for sample preparation) with soap (or any polymeric detergent). Always, rinse your glassware with hot water and then an organic solvent like 70% ethanol or methanol.
- Freshly prepare all the buffers for MS analysis.
- CRITICAL: : Mass spectrometry is highly sensitive to various contaminants, such as PEG (polyethylene glycol), keratin and various salts. Therefore, it is recommended to use powder free nitryl gloves and work in a dust- free environment.
- CRITICAL: The preferred MS compatible protein extraction buffer, ideal for optimum trypsin activity, is 50 mM ammonium bicarbonate buffer. If you had used any other buffer for washing or re-suspending the cell pellets (or tissues), desalting (or dialysis) and buffer exchange (using 50 mM ammonium bicarbonate) should be performed. It can be accomplished using a centrifugal filter with an appropriate molecular-weight-cut-off (MWCO) membrane such as Amicon Ultra 0.5 mL centrifugal filters having 3 kDa MWCO (P/N : UFC500324, Merk Millipore). Please supply the protein sample in 50 mM ammonium bicarbonate buffer.
-
CRITICAL: The commonly used detergents are NOT compatible with
MS. These include NP-40, Triton, CHAPS, SDS (sodium dodecyl sulfate)
and LDS (Lithium dodecyl sulfate), Octyl glucoside, and octyl
thioglucoside, sodium deoxycholate, lauryl maltoside, Brij-35, etc.
If you have to use detergent for the cell lysis, use only
MS-compatible detergents. A few MS-compatible detergents are listed
below.
-
RapiGest SF from Waters
https://www.waters.com/nextgen/in/en/shop/standards--reagents/186001861-rapigest-sf-1-mg--5-pk.html -
PPS Silent Surfactant from Agilent
https://www.chem-agilent.com/pdf/strata/400500.pdf -
Detergent-free buffer from Sigma
https://www.sigmaaldrich.com/IN/en/technical-documents/protocol/protein-biology/protein-lysis-and-extraction/extraction-from-tissue
-
RapiGest SF from Waters
- After desalting and buffer exchange, do protein quantification. The sample (protein) has to have a concentration of 1 mg/mL and 100 microlitres is required (100 micrograms in 100 microlitres). If the concentration of your sample is less than 1 mg/mL, let us know in advance.
- If you have samples to be compared (for relative protein quantification), all samples should be in uniform concentration and volume.
- We suggest sharing the protein isolation protocol (which you intend to follow) with the facility staff and getting their approval before proceeding with the sample preparation.
- Shipment:The samples need to be shipped in a frozen condition in dry ice or brought to RGCB in ice by hand.
In-Solution Sample Digestion Protocol
Reagents Required
| 1 | Ammonium bicarbonate (Sigma A6141) | working concentration 50 mM |
| 2 | Dithiothreitol (Sigma D5545) | working concentration 100 mM |
| 3 | Iodoacetamide (Sigma I1149) | working concentration 200 mM |
| 4 | MS grade Trypsin (Sigma T6567) | working concentration 0.4μg /μl |
| 5 | Formic acid (Merck 5.33002.0050) |
Reagent Setup (prepare freshly before use)
- DTT solution: 100 mM DTT in 50 mM ammonium bicarbonate.
- Iodoacetamide solution: 200 mM Iodoacetamide solution in 50 mM ammonium bicarbonate.
- Formic acid 1% in milliQ water (v/v).
- Trypsin buffer: 0.4 μg /μl trypsin in 50 mM ammonium bicarbonate.
Trypsin digestion protocol for 100 μg of protein sample
- Add 5μl of 100 mM DTT solution to the protein sample.
- Incubate at 60° Celsius for 30 minutes
- Add 5 μl of 200 mM Iodoacetamide solution to the protein sample.
- Incubate at room temperature in dark for 30 minutes.
- Add trypsin buffer in the ratio of 1:25 (1μg of trypsin to 25μg of protein).
- Incubate at 37° Celsius overnight with gentle shaking.
- To arrest the reaction, add 1 μl of 1% formic acid (v/v) to the samples.
- Incubate at 37° Celsius for 20 minutes.
- Store the samples at -20 deep freezer until use.