Research Summary
A similarity has been established in the type of work we do in the lab to the initials of my name. Its DD and it stands for DEVELOPMENT to DISEASE. The focus of our lab is to study mammalian development, get the cues from these studies and thereby apply them to understand the disease biology that actually impact the human being. We understand the we are aiming higher but until and unless you dream of something, how or what will drive us to achieve that dream...We use different models- right from prokaryotic system to cell line to mouse/rat and of course human patient samples. So, the research performed in our lab could be broadly classified into-
- Development (including pre-implantation, post-implantation, hematopoiesis, pluripotency)
- Disease modeling (breast cancer, leukemia)
The current focus area for ongoing research include:
- Histone epigenetic in defects of pre- and post-implantation of embryo and pre-eclampsia
- Regulation of chromatin architecture in hematopoiesis vs leukemogenesis
- Stem cells and centrosome
- Evaluation of histone epigenetic factors as biomarkers for breast cancer and pre-eclampsia
- Biomanufacturing- cultivated meat as smart protein
Presently accepting PhD students under these 3 themes:
- States of pluripotency
- Centrosome regulation in development and disease
- Women reproductive Health
Research Programs
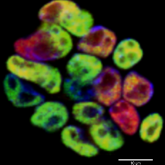
Embryonic fibroblasts upon transduced with transcription factors Oct4, Klf4, Sox2 and c-Myc generated induced pluripotent stem cells (iPSCs) similar in characteristics to embryonic stem cells (ESCs). Till now, they have been derived from different species and from other somatic cell populations with varied protocols. Their derivation is ethically and legally less problematic and technically more feasible. Reprogramming cells to a pluripotent state entails global epigenetic remodelling and introduces epigenetic changes, some of which are necessary for reprogramming to occur and others of which are inadvertently introduced during the process. Generating patient specific stem cells has been a long-standing goal in the field of regenerative medicine. These cells provide a unique platform from which to gain mechanistic insight into a variety of diseases, to carry out in vitro drug screening, to evaluate potential therapeutics and to explore gene repair coupled with cell replacement therapy. We will focus on the transcription factors and epigenetic modifications that could regulate induction of pluripotency. With a better understanding on the culture condition for reprogramming differentiated cells, we will efficiently derive human iPSCs suitable for clinical applications in the field of cardiovascular diseases.
Pluripotent factors (OCT4/NANOG) in iPSC colony
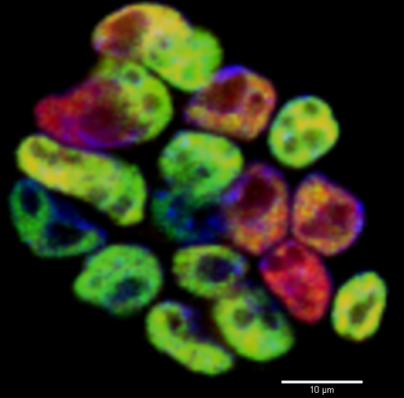
Eukaryotic DNA is organized into chromatin in a dynamic manner that enables it to be accessed during different cellular processes. Histones, the chief protein component of chromatin, must be assembled, replaced or exchanged to preserve or change this organization according to the cellular requirements. Recent genetic studies indicate that specific histone modifications and modifying enzymes play essential roles in both global and tissue-specific chromatin organization. In particular, these studies indicate that enzymes that control levels and patterns of histone acetylation and methylation are required for normal embryo patterning, organogenesis, and survival. So we will focus on the role of these factors in different context of developmental biology.
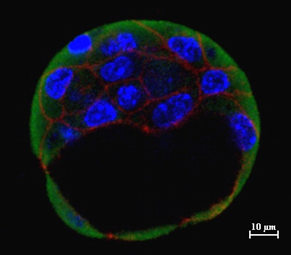
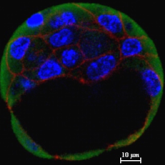
Pre-implantation E3.5 blastocyst demonstrating the expression of E-Cadherin and histone chaperone
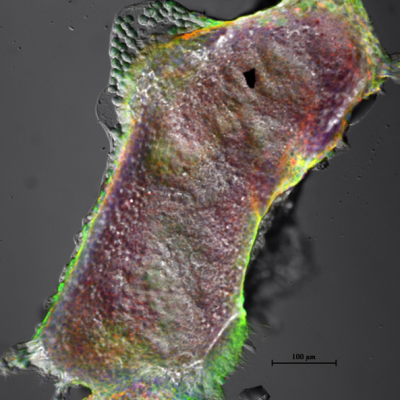
Post-implantation E5.5-E7.5 mouse embryo demonstrating the expression of E-Cadherin and histone chaperone
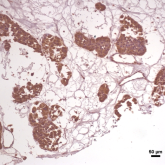
We are focussing in different epigenetic factors that could regulate or dictate the invasive or metastatic character of breast cancer. Tissues, human cell line followed by animal model experimental evidences are used as model to investigate upon these factors.
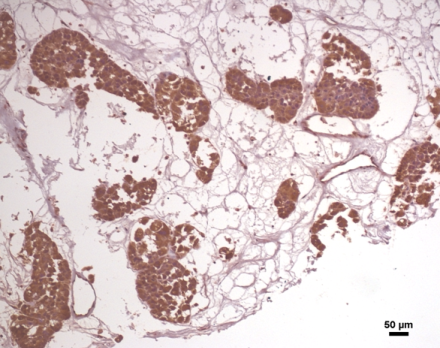
Expression of APLF in breast IDC patient sample
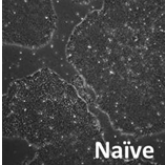
Two states of pluripotency exist for embryonic stem cells, primed and naive. Unfortunately, the human ES cells generally exist in the primed state that makes it difficult to culture them and most importantly for their clonal expansion. So, it is very much needed to convert them or to derive them in naive state to maximize the potential use of the hES cells for regenerative purpose. So, we are focussing on ES cells from different origins to investigate upon the molecular mechanism and the way out to develop naive human ES cells.
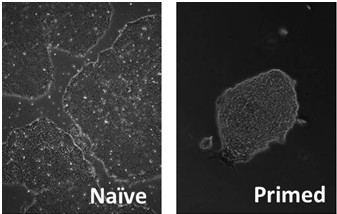
Naive vs. primed state of pluripotency

With biggest population of the world, India is amongst the largest consumption of meat and dairy products and with time, feeding this demand would be difficult. This will create a significant pressure on natural resources. Livestock production accounts for 80% of global greenhouse gas emissions and uses 83% of farmland. Additionally, 60% of known and 75% of new infectious diseases threatening humans come from animals. Hence, efforts are being made for large scale dietary modifications that are likely to lessen these consequences. Hence, a sustainable alternative is very important to be harnessed and processed that could serve the population its need for a healthy diet. Generation of smart proteins by harnessing the resources of the country will provide the sustainable indigenous alternative proteins that could feed India and also the world.
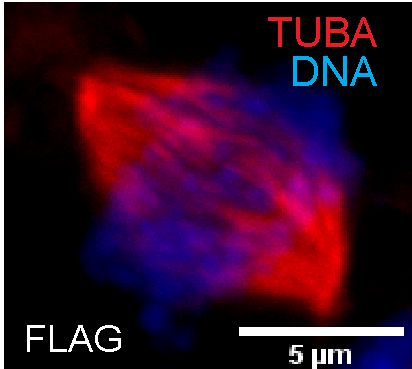
Centrosome duplication and its number undergoes change or aberration during disease like breast cancer or in propagation of embryonic stem cells (ESCs) leading to genomic instability. We are investigating the role of histone chaperone APLF, also a kinase, on its regulation in controlling the centrosome duplication and its number and thereby its role in development and disease.

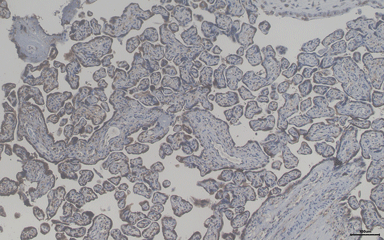
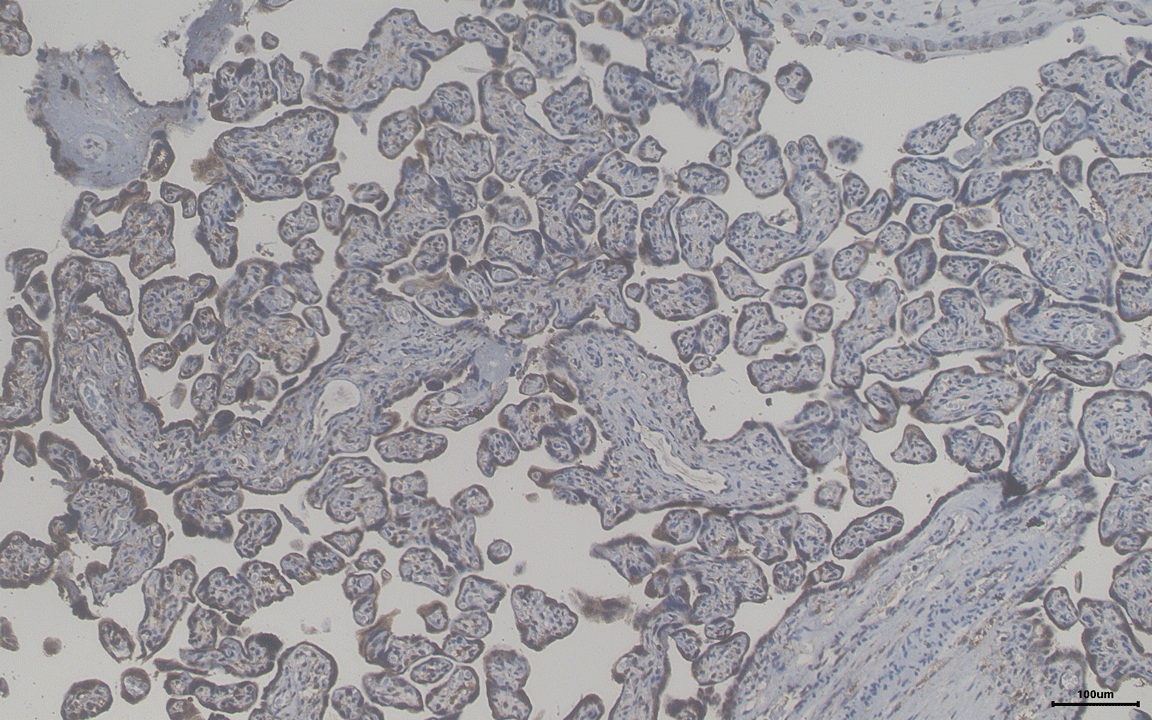
Our studies on pre-implantation development demonstrated the role of histone chaperone in implantation. Accordingly, we are looking into how this molecule could modulate the maternal reproductive health associated diseases like preeclampsia. We are analysing blood and placenta samples from pregnant women (normal and preeclampsia) to investigate upon the role of this molecule as a biomarker. We are also trying to understand the molecular mechanism that is involved in the induction of pregnancy associated disorders in absence or presence of histone chaperone APLF by exploiting the models of blastoid or gastruloid generated from embryonic stem cells and trophoblast stem cells.
Current Research Grants
-
2028 2026
Formulation of serum free media
DBT-BIRAC -
2027 2024
Designing a kinase inhibitor cocktail in regulation of breast cancer metastasis
CSIR ASPIRE -
2027 2024
Centrosome duplication in stem cells and development,
Department of Biotechnology [DBT] -
2026 2023
Dissecting domains of APLF chaperoning EMT in development.
Science and Engineering Research Board [SERB] -
2026 2023
Epigenetic regulation of trophoblast stem cells
Indian Council of Medical Research [ICMR]
Previous/ Completed Research Grants
-
-
States of pluripotency
Department of Biotechnology [DBT] 2017-2019 -
Hemogenic Endothelium
Department of Science & Technology [DST] 2013-2016 -
Transcriptional regulation of VEGFR3
Council of Scientific and Industrial Research [CSIR] 2013-2016 -
Histone chaperone in pluripotency
Department of Biotechnology [DBT] 2012-2015 -
Normal vs. abnormal hematopoiesis
Science and Engineering Research Board [SERB] 2022-2025 -
Metastatic Breast cancer
Department of Biotechnology [DBT] 2018-2023 -
Cellular transition
Department of Biotechnology [DBT] 2017-2020 -
HIRA in proliferation vs differentiation
Department of Science & Technology [DST] 2017-2020
-